If your concentrations of salt are different then you can scale the boiling point elevation and melting point depression predictions directly with the concentration. What is this temperature in degrees Celsius.
 The Boiling Point Of Water Is 100 C Express This In Si Units Kelvin Scale Youtube
The Boiling Point Of Water Is 100 C Express This In Si Units Kelvin Scale Youtube
As defined earlier atmospheric pressure or air pressure is the force applied against a liquids surface.
What is the boiling point of water in celsius degrees. 100 C is the boiling point of water or 212 The freezing point of water is 32. Celsius also called centigrade scale based on 0 for the freezing point of water and 100 for the boiling point of water. Because frozen pipes block water flow it is not possible for tap water to be any colder than freezing.
Please share our efforts. Generally speaking the boiling point is 100 degrees Celsius or 212 degrees Fahrenheit. Invented in 1742 by the Swedish astronomer Anders Celsius it is sometimes called the centigrade scale because of the.
Despite that you need to boil your water for at least ten minutes in temperatures of 60 degrees Celsius. If the heat ever reached a point that the pipes would cause the water to boil it also would not be possible to have water flow. The Celsius scale sets the freezing point and boiling point of water at 0C and 100C respectively.
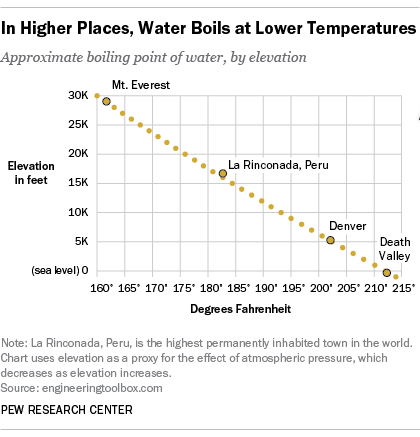
762 m 207 ºF. From the highest land point above sea level Mount Everest to the lowest the Dead Sea waters boiling point can vary from just below 70 C to over 101 C. The boiling point of water depends on the atmospheric pressure which changes according to elevation.
What is the Boiling Point of Water. Tap water temperature is much like room temperature in that it depends on the local conditions. What is the boiling and freezing point of water in Celsius.
The simple answer to this question is that the boiling point of water is 100 C or 212 F at 1 atmosphere of pressure sea level. 0 m 212 ºF. What is the freezing and boiling point of water on the Celsius scale.
However the value is not a constant. The Fahrenheit scale defines the freezing point of water as 32F and the boiling point as 212F. Doing this will eliminate the disease-causing microorganisms and bacteria to have purified drinking water you should also adhere to the time you should boil water for what kind of bacteria and at what level of temperature required for each organism hence have safe.
The boiling point of water at standard atmospheric pressure is 212ºF 100ºC. 457 m 209 ºF. There are 100 degrees between the freezing 0 and boiling points 100 of water on the Celsius scale and 180 degrees between the similar points 32 and 212 on the Fahrenheit scale.
610 m 208 ºF. Water boils are 100 degrees Celcius 212 Fahrenheit. The boiling point is raised by 05 degrees Celsius for water with 292 grams of salt dissolved in each kg of water.
The boiling point of water is 212 degrees Fahrenheit or 100 degrees Celsius but only at sea level. The reason for this variation comes down to the differences in atmospheric pressure at different elevations. The Celsius scale sets the freezing point and boiling point of water at 0C and 100C respectively.
What is the boiling point of water. Boiling Point - Fahrenheit. The boiling point of water is 212F.
Boiling point is the temperature at which the saturated vapor pressure of a liquid is equal to standard atmospheric pressure at sea level. 1067 m 2055 ºF. Updated October 06 2019.
The boiling point of water is 100C 212F or 37315 Kelvin under standard conditions at sea level at one atmosphere of pressureThe boiling point of water. When liquid is heated to the boiling point the liquid will evaporate into the air. 1000 ft 305 m 210 ºF.
152 m 211 ºF. 3000 ft 914 m 206 ºF. As a thumb rule the higher the atmospheric pressure the more heat energy is necessary to boil a liquidor in this case water.
At altitude water boils at 934 C 2001 F at 1905 meters 6250 ft. 1219 m 204 ºF. Boiling Point - Celsius.
Use the formula C 59F 32 where C represents the temperature in degrees Celsius and F represents the temperature in degrees Fahrenheit.
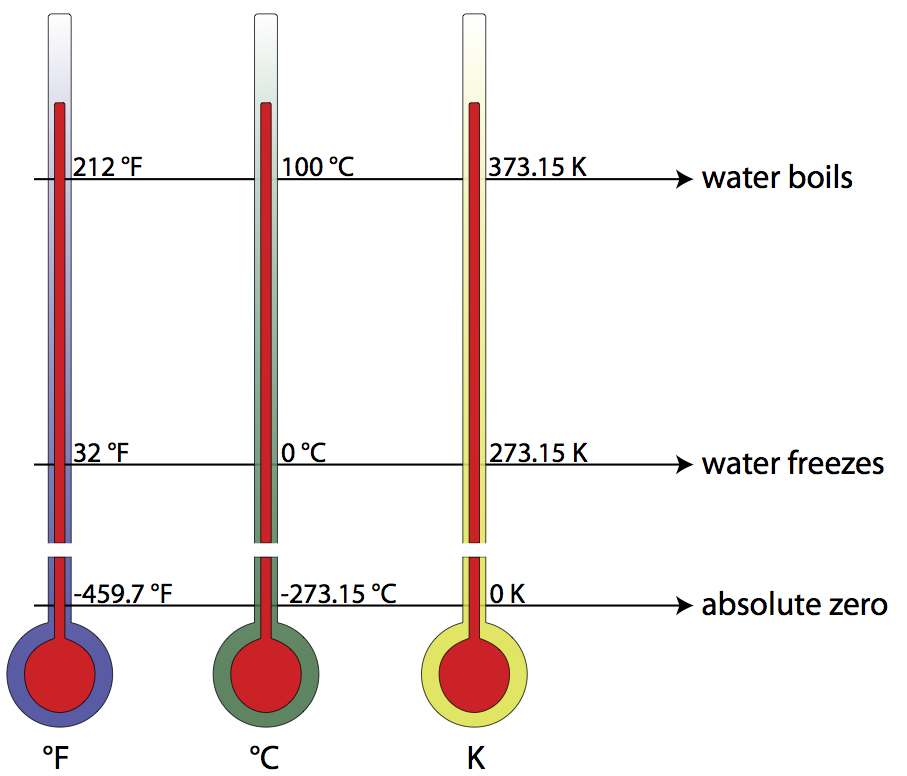
Physicists have now created an atomic gas in the laboratory that nonetheless has negative Kelvin values. Hence the two reference temperatures for Celsius the freezing point of water 0C and the boiling point of water 100C correspond to 27315K and 37315K respectively.
 Temperature V Heat Celsius Kelvin Temperature Scales Boiling Point Of Water Freezing Point Of Water Absolute Zero Celsius 100 Celsius Degrees Ppt Download
Temperature V Heat Celsius Kelvin Temperature Scales Boiling Point Of Water Freezing Point Of Water Absolute Zero Celsius 100 Celsius Degrees Ppt Download
The scientific community often uses kelvin and Celsius measurements interchangeably or at the same time.
Boiling point of water kelvin. 7 1 Temperature And Pressure Chemistry Libretexts. The boiling point of water in kelvin scale is 37315 K. The boiling point of water is 37315 degrees Kelvin K.
Hence the two reference temperatures for Celsius the freezing point of water 0C and the boiling point of water 100C correspond to 27315K and 37315K respectively. Jan 182021 - What Is the boiling point of water at Celsius and kelvin. What is the boiling point of water at 1 atm in Kelvin - 15938605.
Click to see full answer. T 10027315 37315K. It seems like one of those basic science facts.
The Kelvin degree is the same size as the Celsius degree. Water boils at a lower temperature as you gain altitude eg going higher on a mountain and boils at a higher temperature if you increase atmospheric pressure coming back down to sea level or going below it. The boiling point of water in Kelvin is 373 K.
The boiling point of water in kelvin 100273 373 Kelvin and in degree Celcius it is 100. Boiling point of water. Also the Melting and Freezing point of water in Kelvin is.
Hence by T K toC 27315. 1K 27315 -2721C Image will be Uploaded Soon Now that we know what does kelvin mean let us discuss about what is the Celsius. The Kelvin degree is the.
The Kelvin degree is the same size as the Celsius degree. The Kelvin degree is the same size as the Celsius degree. One may also ask what is the melting point and boiling point of water in Kelvin scale.
Let us discuss what is the formula of Kelvin. A temperature scale called the Reaumur R scale is defined by identifying the freezing point of water as 00R. The boiling point of water depends on the atmospheric pressure which changes according to elevation.
What is the Freezing Point of Water in Kelvin. This is also equivalent to. You may see data on temperature given both a.
Using the Fahrenheit scale waters boiling point is 212 degrees. As boiling point of water in celcius scale is 100 degree celcius. A single kelvin is referred to as a unit rather than a.
The boiling point of water is 373 K in kelvin unit. Water boils at 212 degrees Fahrenheit 100 degrees Celsius right. What is 1 Kelvin.
However the value is not a constant. As such in the Kelvin scale water freezes at 27315 K 0 C and boils at 37315 K or 100 C. Chemistry 11122019 1515 sana1234544 What is the boiling point of water in kelvin scale.
Lord Kelvin 212F. The boiling point of water approximately 100 C or 212 F is exactly 3731339 K. The boiling point of water is 100oC.
It is known that the boiling point of water on the Kelvin temperature scale is 37315 K and the freezing temperature of water is 27315 K. So as per formula K27315c. The freezing point of water in Kelvin is 273 K.
Hence the two reference temperatures for Celsius the freezing point of water 0C and the boiling point of water 100C correspond to 27315K and 37315K respectively.
The boiling point of a liquid is a characteristic property and can be treated as criteria for the purity of liquid. These are the colligative properties that depends only on the no.
 Temperature And Temperature Scales Chemistry For Non Majors
Temperature And Temperature Scales Chemistry For Non Majors
A solute lowers the freezing point of a solvent.
Boiling point and freezing point. 2 The depression in freezing point and the elevation in boiling point increases with increase in the concentration of the solute or impurity ie. Boiling Point and Freezing Point. The normal boiling point of water is 100 o C because this is the temperature at which the vapor pressure of water is 760 mmHg or 1 atm.
This week we will be identifying the boiling point of water and the freezing melting point of water. In fact as the boiling point of a solvent increases its freezing point decreases. September 11 2014.
Similarly freezing point depression is the lowering of a solvents freezing point due to the addition of a solute. Boiling Point of solution normal boiling point of solvent ΔT b. The freezing point is lowered while the boiling point is raised.
One may also ask what is melting point boiling point and freezing point. I cant tell you how surprised I was the first time I boiled water for my fifth graders. The boiling point is the temperature at which a material changes from a liquid to a gas boils while the melting point is the temperature at which a material changes from a solid to a liquid melts.
Of moles of the solute. ΔT f -K f m ΔT f the amount by which the freezing point is lowered. In dilute solutions the freezing point depression is proportional to the molality of the solute particles.
Boiling point is defined as the temperature at which the vapour pressure of the liquid becomes equal to the atmospheric pressure. Consequently measuring one of these properties for a solution prepared using a known mass of solute permits determination of the solutes molar mass. At at high altitudes the lower pressure makes the boiling point several degrees lower.
Increasing the glycerine concentration above 667 will increase the freezing point as indicated below. Glycerine to Water Concentration. The first two physical properties we will cover are boiling and freezing point the point at which a substance turns into a gas and the point at which it tu.
The bag of the salt became colder so the mixture froze faster. Boiling Point of a Liquid. The boiling points of glycerine also called glycerin or glycerol water mixtures are reduced with increased amounts of glycerine.
Generally there are three states of an object. The freezing points are reduced until glycerine concentration is 667 mass. For pure water the boiling point is 100 degrees Celsius 212 Fahrenheit at one atmosphere of pressure and the melting point is 0 degrees Celsius 32 degrees Fahrenheit at one atmosphere of pressure.
However the amount to which the boiling point increases or the freezing point decreases depends on the amount of solute that is added to the solvent. Keep in mind that a materials melting point is the same as its freezing point. Boiling water is no big deal to us grown-ups because we see it almost every time we cook.
Under normal conditions when the pressure of the atmosphere is approximately 760 mmHg water boils at 100 o C. Osmotic pressure and changes in freezing point boiling point and vapor pressure are directly proportional to the number of solute species present in a given amount of solution. The boiling point of a solution is higher than the boiling point of a pure solvent and the freezing point of a solution is lower than the freezing point of a pure solvent.
It increases with the increase in external pressure. Thus as solutes or solids are applied to liquids the freezing point drops and the boiling point rises. Boiling point elevation is the raising of a solvents boiling point due to the addition of a solute.
In principle the boiling-point elevation and the freezing-point depression could be used interchangeably for this purpose. 1 The impurities present in a liquid pull its two fixed points away from each other ie. The boiling point always occurs for a liquid it is the temperature at which the liquid.
Elevation of Boiling Point. Solid liquid and vapor. The freezing point of a pure solvent is lowered by the addition of a solute which is insoluble in the solid solvent and the measurement of this difference is called cryoscopyIt is found that Can also be written as Here K f is the cryoscopic constant equal to 186 C kgmol for the freezing point of water i is the van t Hoff factor and m the molality.
Since liquid salt decreases the freezing point of water the temperature of the bag of salt drops below zero degrees -1 degree Celsius. However the cryoscopic constant is larger than the ebullioscopic constant and the freezing point is often easier to measure with precision which means measurements using the freezing-point depression are more precise.
The amount of energy needed to raise the temperature of 1g of water by 1 degree Celsius or 1 Kelvin equals 1 calorie. The kelvin is now defined by fixing the numerical value of the Boltzmann constant k to 1380 64910 23 JK 1.
 What Is The Freezing Point Of Water Fahrenheit Celsius And Kelvin
What Is The Freezing Point Of Water Fahrenheit Celsius And Kelvin
1C 27315 27415 k.

Kelvin boiling point of water. Click to see full answer. Temperature scale in which zero occurs at absolute zero and each degree equals one kelvin. A human body temperature.
Find the value of 50F in Kelvin. Boiling Point Of Water At Sea Level In Kelvin - Hi Guys Awesome Sea On this occasion we gave several images wallpapers related to the title Boiling Point Of Water At Sea Level In Kelvin you can download it for reference or collection. The boiling point of water is 37315 degrees Kelvin K.
What is the Freezing Point of Water in Kelvin The freezing point of water in Kelvin is 273 K. The temperature for the Kelvin scale. 0 represented the boiling point of water while 100 represented the freezing point of water.
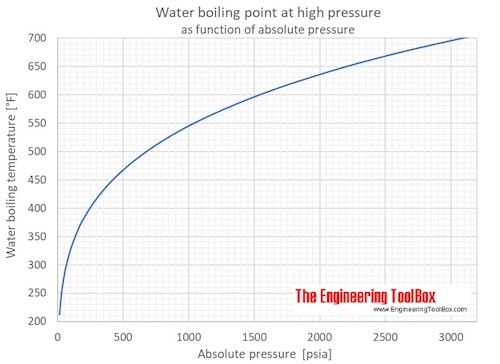
Water freezes at 27315 K and boils at 37315 K. The Kelvin degree is the same size as the Celsius degree. The Boiling temperature of water for atmospheric pressure using Antoine equation is the temperature at which the vapour pressure is equal to the standard sea-level atmospheric pressure and is represented as bp 173063807131-log10 P atm-233426 or boiling_point 173063807131-log10 Atmospheric Pressure-233426.
Atmospheric pressure also known. Click to see full answer. The boiling point of water approximately 100 C or 212 F is exactly 3731339 K.
The boiling point of water in kelvin scale is 37315 K. Hence by T K toC 27315. You may see data on temperature given both a.
The melting point of water in kelvin. What is the Boiling Point of Water in Kelvin The boiling point of water in Kelvin is 373 K. Answer begintabularlcc Celsius kelvin.
10C 27315 28315 k. It is named after the Belfast-born Glasgow University engineer and physicist William Thomson 1st Baron Kelvin 18241907. A calorie is a unit of measuring energy.
T 10027315 37315K. The boiling point of water is 100oC. Hence the two reference temperatures for Celsius the freezing point of water 0C and the boiling point of water 100C correspond to 27315K and 37315K respectively.
Determine the kelvin temperature for each of the following. Hence the two reference temperatures for Celsius the freezing point of water 0C and the boiling point of water 100C correspond to 27315K and 37315K respectively. This is also equivalent to.
Assuming that waters room temperature before boiling is 25 0 C and since the boiling point of water is 100 0 C you would need to raise the temperature of water by 100 0 C-25 0 C75 0 C. Find the value of 41F in Celsius. Using the Fahrenheit scale waters boiling point is 212 degrees.
B the boiling point of water at the standard pressure of 1 atm. Boiling Freezing Melting point of water in kelvin 0F 1778C. Temperature scale that registers the freezing point of water as 0 degrees C and the boiling point as 100 degrees C under normal atmospheric pressure.
In 1742 Swedish astronomer Anders Celsius 17011744 created a temperature scale that was the reverse of the scale now known as Celsius. So as per formula K27315c. 0 k 27315C.
The kelvin is the base unit of temperature in the International System of Units SI having the unit symbol K. C the coldest day you can remember. Boiling point of water.
As boiling point of water in celcius scale is 100 degree celcius. The scientific community often uses kelvin and Celsius measurements interchangeably or at the same time. The Kelvin degree is the same size as the Celsius degree.
D the boiling point of liquid nitrogen left-196circ mathrmCright e the melting point of lead left327circ mathrmCright. In his paper Observations of two persistent degrees on a thermometer he recounted his experiments showing that the melting point of ice is.
What is the boiling and freezing point of water in Celsius. When the altitude increases the boiling point of water decreases.
/BoilingWater-58dd1c2a5f9b5846837d2a23.jpg) What Is The Boiling Point Of Water
What Is The Boiling Point Of Water
In this experiment you will measure the boiling point of two unknown liquids.
Boiling point of water in celsius. In this regard the boiling point of water changes with a change in barometric pressure. 762 m 207 ºF. The Fahrenheit scale defines the freezing point of water as 32F and the boiling point as 212F.
For a 10 molal solution of salt containing 5844 grams of salt per kg of water the boiling point is raised by 10 degrees Celsius. Actually the formula for boiling point uses this value as the basis of calculations. It is always the same - 100C or 212F.
1067 m 2055 ºF. 3000 ft 914 m 206 ºF. At 24384 metres 8000 ft in elevation water boils at just 92 C 198 F.
The boiling point for water at sea level and under standard conditions is 100 degrees Celsius 212F. The boiling point of salt water depends on the amount of salt added. The boiling point of water depends on the atmospheric.
If you really care the mystery fluids are water and isopropyl alcohol. Boiling as a cooking method must be adjusted or alternatives applied. The normal boiling point is the temperature at which the vapour pressure is equal to the standard sea-level atmospheric pressure 760 mm 2992 inches of mercury.
Changes in atmospheric pressure will alter the temperature at which water boils. What is the Boiling Point of Water. Conventionally the temperature at which water boils is 100 degrees Celsius or 212 Fahrenheit but only at sea level.
The boiling point of a liquid varies according to the applied pressure. Similarly the freezing point of water at sea level is a constant value -. You dont have to use our boiling point at altitude calculator to determine the boiling point of water at sea level.
Celsius is a unit of temperature scale and it is represented by C pronounced as degree celsius degree cel-see-uhs In some countries Celsius is also known as centigrade. The freezing and boiling points of water were part of the definition of the Celsius scale for a long period of time 1743-1954. Boiling Point - Fahrenheit.
The Celsius scale sets the freezing point and boiling point of water at 0C and 100C respectively. It is 212 degrees F and 100 Celsius. It was redefined in 1954 to be based on absolute zero and the triple point of water which related the Celsius scale precisely to the Kelvin scale.
610 m 208 ºF. 1000 ft 305 m 210 ºF. 1219 m 204 ºF.
0 m 212 ºF. 457 m 209 ºF. However the value is not a constant.
British Standard 6008 and International Standard ISO 3103 advise that tea is best made with water that is freshly boiled. Water boils at 212F at sea level but only at sea level. 152 m 211 ºF.
The temperature scale that represents the boiling point of water as 100 C and freezing point of water as 0 C is defined as celsius scale. The Celsius scale sets the freezing point and boiling point of water at 0C and 100C respectively. For every 1524-metre 500 ft increase in elevation waters boiling point is lowered by approximately 05 C.
100 - the boiling point of water. At sea level water boils at 100 C 212 F. At sea level water boils at 100 C 212 F.
The Fahrenheit scale defines the freezing point of water as 32F and the boiling point as 212F. 0 - the freezing point of water. The simple answer to this question is that the boiling point of water is 100 C or 212 F at 1 atmosphere of pressure sea level.
Boiling Point - Celsius. For pure water the boiling point is 100 degrees Celsius 212 Fahrenheit at one atmosphere of pressure and the melting point is 0 degrees Celsius 32 degrees Fahrenheit at one atmosphere of pressure. Updated October 06 2019.
At at high altitudes the lower pressure makes the boiling point several degrees lower. The boiling point of water is well-known to you. At higher altitudes the temperature of the boiling point is.
Boiling point of water.
ads
