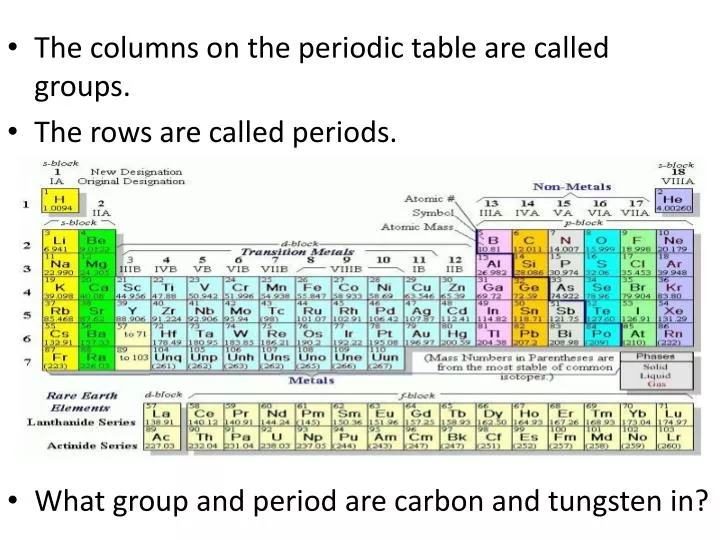
The groups are numbered one through 18. There are 18 Groups on the Periodic Tableand the Group number refers to the number of valence electrons.
How Many Columns Are In A Periodic Table Quora
The elements in a group share the same configuration of valence electrons which gives the elements similar chemical properties.
Columns of the periodic table. Because alkali metals __________ quickly with other elements they exist in nature only as _________. Groups 3 to 12 of the periodic table. As well as being numbered some of these groups have namesfor example alkali metals the first column of elements alkaline earth metals the second column of elements halogens the next-to-last column of elements and noble gases the last column of elements.
Groups indicate elements with similar chemical and physical properties. Also noble gas element in group 18. Group 2A of the periodic table.
Usually individual groups bear the name of the lightest metal eg Vanadium group. Elements within the same period or group have similar properties. Group 8A of the periodic table.
The groups of the periodic table are displayed as vertical columns numbered from 1 to 18. From Wikipedia the free encyclopedia In the periodic table of the elements each numbered column is a group. The Elements displayed in each Periodic Table Group are either Gas Liquid or Solid at room temperature and are classified in groups as.
Determining Chemical Properties using the Periodic Table. Group 7A of the periodic table. Elements in the same group can be expected to behave in a similar way because they have the same number of electrons in.
The older IUPAC system used Roman numerals together with letters to distinguish between the left A and right B side of the periodic table. The columns of the modern periodic table represent groups of elements and rows represent the periods. A vertical column in the periodic table.
Herein how are elements classified into groups. The periodic table is the tabular arrangement of all the chemical elements on the basis of their respective atomic numbers. Each row of the periodic table is called a period and each column of the periodic table is called a group or family.
Inner transition metal in the bottom of the bottom two rows of the periodic table. Element in group 2. The columns on the periodic table of elements are called groups.
The modern periodic table is based on the modern periodic law put forward by the English physicist Henry Moseley which states that. Some groups have specific names like the halogens or noble gases. Group 1A of the periodic table.
Element in group 17. We now know elements in the periodic table are ordered by atomic numbers. The elements in a group have very similar chemical properties which arise from the number of valence electrons presentthat is the number of electrons in the outermost shell of an atom.
The modern Periodic Table reflects electronic structure. Vertical column of the periodic table. The columns that comprise the periodic table are called groups -- 18 in total.
Here are some of the names for the various groupe columns 1st Li NaK. Element in group 1. Alkaline earth metals 3rd to 10th.
The f-block columns between groups 2 and 3 are not numbered. The number of valence electrons in a group is sometimes represented with a Roman numeral above the column. A group is a vertical column in the periodic table of the elements.
In the periodic table the vertical columns are called groups and the horizontal rows are called periods. In chemistry a group also known as a family is a column of elements in the periodic table of the chemical elements. The elements in group 1 of the periodic table are the _________ They include lithium sodium potassium rubidium cesium and francium.
Elements typically metals can lose electrons to become oxidized or typically as non-metals can gain electrons to become anions. A column of elements on the periodic table. Alkali Metals Alkaline Earth Metals Transition Metals Metalloids Other Metals Non-metals Halogens Noble Gases and Rare Earth Elements.
There are 18 numbered groups in the periodic table. Alkali metals 2nd Be Mg Ca. Element in group 16.
Columns of the periodic table typically mark groups or families. Apart from a few instances such as tellurium and iodine which have caused considerable confusion this order is the same as the order of elements relative atomic masses. Groups are considered the most important method of classifying the elements.
Three systems have been used to number families and groups. About 80 percent of the elements are metals shiny elements that conduct heat and electricity well and 15 percent of the elements are nonmetals poor conductors of heat and electricity.
ads
