Plains have mostly flat land covered with grass and a only a few trees. The state of Nebraska can be found in the central regions of the United States enclosed by the states of Kansas Colorado Wyoming South Dakota and Iowa.
 Nebraska Capital Map Population History Facts Britannica
Nebraska Capital Map Population History Facts Britannica
The western edge of the city lies in the valley of Salt Creek which flows northeastward to the lower Platte River.
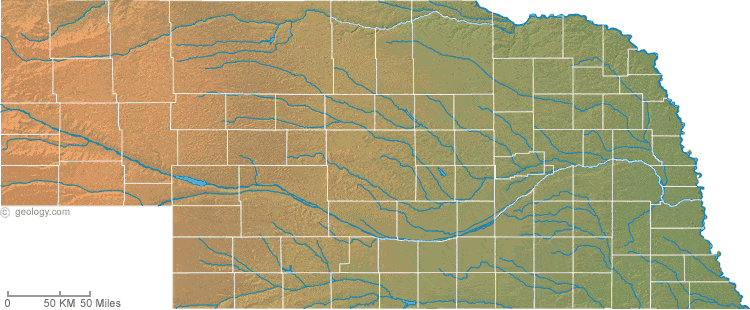
Physical features of nebraska. Access Nebraska almanac furnishing more details on the state geography geographical and land regions climate and weather elevation land areas bordering states and other statistical data. The upward slope of the terrain to the west causes instability in moist easterly winds. D PROFESSOR OF NATURAL SCIENCES IN THE UNIVERSITY OF NEBRASKA.
Nebraska is composed of two major land regions. Nebraska is bordered by South Dakota on the north and by Colorado and Kansas on the south. All many people know of Nebraska is cornfields.
Set near the center of Lancaster County in southeastern Nebraska Lincoln is surrounded by gently rolling prairie. Missouri River at 840 feet located in the countysubdivision of Richardson source. State Abbreviation - NE State Capital - Lincoln Largest City - Omaha Area - 77358 square miles Nebraska is the 16th biggest state in the USA Population - 1868516 as of 2013 Nebraska is the 37th most populous state in the USA Name for Residents - Nebraskans Major Industries - farming corn.
The most important geographic features of Grand Island are the. Articles Animals Fine Arts Language Arts Places Plants and Other Living Things Science and Mathematics Social Studies Sports and Hobbies World. The state is characterized by the Missouri river flowing on the eastern border and its tributaries that run from the High Plains on the west towards Missouri on the east.
Written by William Cutler and published by Andreas in 1882 this extraordinary book provides a detailed and comprehensive history of Nebraska. Nebraska is in the Gulf of Mexico Watershed and the topography is a plain that gently slopes to the east. Andreas History of the State of Nebraska - Physical and Natural Features - Part 2.
Geological Survey Central Point. GEOGRAPHY AND LANDFORMS Nebraska is bordered by South Dakota in the north Iowa and Missouri in the east Kansas in the south Colorado in the south and west and Wyoming in the west. Its a reputation that simply isnt fair.
Physical map Physical map illustrates the mountains lowlands oceans lakes and rivers and other physical landscape features of Nebraska. Produced by Gary Martens and Connie Snyder. Panorama Point at 5424 feet located in the countysubdivision of Kimball source.
PHYSICAL AND NATURAL FEATURES. The Great Plains region occupying most of western Nebraska is characterized by treeless prairie. The Dissected Till Plains region consist of gently rolling hills and contains the states largest cities Omaha and Lincoln.
The Sand Hills region of north-central and northwestern Nebraska is one of the states most distinctive features. Differences in land elevations relative to the sea level are represented by color. Most of these lakes and streams can be clearly seen on the Nebraska Satellite Image.
The flat land that Grand Island is built on is called plains. Landforms and Geographic Features of Nebraska Photo Nebraska has a reputation as a flat featureless expanse of dry prairie. The rivers and streams follow this slope and mostly drain into the Missouri River which forms the eastern boundary of the state.
The Geography of Nebraska Total Size. Comprising nearly one-fourth of the area of the state it consists of sloping hills and valleys varying from 25 to 400 feet 8 to 120 metres in elevation. Geological Survey Geographical High Point.
2003 Census Geographical Low Point. Nebraska has a variety of physical features. Nebraska was the 37 th state in the USA.
While the state is made of thousands of acres of just that it also has river valleys bluffs buttes open prairie grasslands and rock formations. TOPOGRAPHY AND GENERAL CHARACTER OF NEBRASKA. These include the Great Plains the Bad Lands as well as The Chimney Rock.
Nebraska Physical Cultural Historic Features and Landmarks with maps driving direction and local resources. The Dissected Till Plains and the Great Plains. Andreas History of the State of Nebraska - Physical and Natural Features.
It became a state on March 1 1867. Although the word flat is.
This formula means that there are two hydrogen atoms and one oxygen atom in each water molecule. Hydrogen peroxide is unstable decomposing readily to oxygen and water with release of heat.
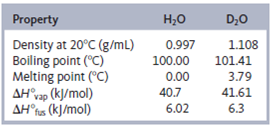 Solved Some Of The Physical Properties Of H2o And D2o Are As Foll Chegg Com
Solved Some Of The Physical Properties Of H2o And D2o Are As Foll Chegg Com
It is colorless odorless and tasteless but heavier than normal water.
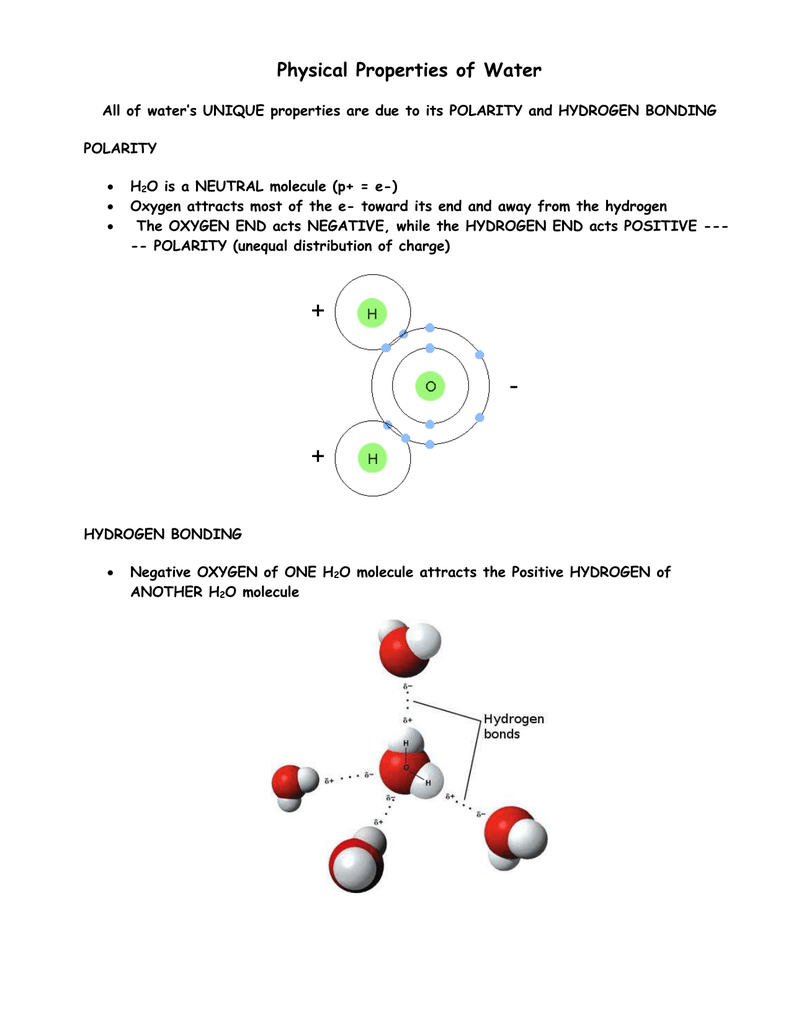
Physical properties of h2o. Physical Properties of Liquid Water Specific Heat c p BTUlbmR Thermal Conductivity k BTUhrftF Viscosity µ lbmhrft Temp F Saturated Liquid 1000 psia 2000 psia Saturated Liquid 1000 psia 2000 psia Saturated Liquid 1000 psia 2000 psia 80 09975 09943 09912 03532 03537 03570 2084 2084 2083. Cohesion is a key property of water. The slightly negative regions of one molecule are attracted to the slightly positive regions of nearby molecules forming a hydrogen bond.
In the air oxygen is simply not distinguishable since under normal conditions it is a gas without taste color and smell. There are several important properties of water that distinguish it from other molecules and make it the key compound for life. The chemical formula of water is H2O.
71 of the earths surface is made up of this liquid. It is made up of two hydrogen atoms and one oxygen atom which are held together by covalent bonds. Each water molecule can form hydrogen bonds with up to four neighbors.
But oxygen can be artificially transferred to other states of aggregation. Gas Steam or Vapor Physical States Vapor States of Water Water molecules are constantly moving Temperature increase Increase in movement Hydrological Cycle States of Water When water molecules move faster they tend to break their hydrogen bonds. However oxygen gas is colourless odourless and tasteless.
Water has a variety of unusual properties because of attractions between these polar molecules. The Physical properties of Oxygen are the characteristics that can be observed without changing the substance into another substance. Physical properties of oxygen.
Hydrogen bonds form between neighboring molecules. The molecular formula of water is H 2 O. Because of the polarity of the molecules water molecules are attracted to each other.
Properties of Saturated Water Presented at Regular Intervals of Pressure Specific volume m3kg Specific internal energy kJkg Specific enthalpy kJkg Specific entropy kJkg-K Pressure P kPa Temp. Physical properties are usually those that can be observed using our senses such as color luster freezing point boiling point melting point density hardness and odor. Ionic compounds are less soluble in heavy water because its dielectric constant is lower than that of H₂O.
The H stands for hydrogen the O stands for oxygen. The liquid and solid forms are a pale blue colour. Physical Properties The molecular mass of heavy water is higher than ordinary water which makes their properties different from each other.
Boiling Point Of Water. Although nonflammable it is a powerful oxidizing agent that can cause spontaneous combustion when it. Small amounts of gaseous hydrogen peroxide occur naturally in the air.
So at -183 o C it becomes liquid and at. T C 103 v f vg uf ug hf hg sf sg P kPa 1 69705 10001 12919 29302 23845 29303 25137 010593 89749 1. Physical Properties Oxygen exists in all three forms - liquid solid and gas.
Hydrogen peroxide is a colorless liquid at room temperature with a bitter taste.
Hence it is essential for plants. Carstensen University of Wisconsin School of.
 Chapter 3 Physical And Chemical Properties Physical And Chemical Changes Ppt Download
Chapter 3 Physical And Chemical Properties Physical And Chemical Changes Ppt Download
Physical Properties of Magnesium Edu-Resource.

Physical and chemical properties of magnesium. Introduction Physical Properties of Magnesium Oxide Chemical Properties of Magnesium Oxide Surface Structures of MgO Molecular Adsorption on MgO Bibli. This element is one of the group 2 and period 3 with the periodic table and contains the atomic number 12. Ertel x Merrell Dow Research Institute Cincinnati OH 45215 Merrell Dow Research Institute Cincinnati OH 45215 x Merrell Dow Research Institute Cincinnati OH 45215 Merrell Dow Research Institute Cincinnati OH 45215 JT.
No differences were observed in the physical state of pipemidic acid and in microsphere shape and surface between different size fractions of microspheres prepared with different amounts of magnesium stearate. Summary This chapter contains sections titled. Most common substances exist as States of Matter as solids liquids gases and plasma.
Magnesium Mg chemical element one of the alkaline-earth metals of Group 2 IIa of the periodic table and the lightest structural metal. Open image in new window. Magnesium is an important.
The element can be found in abundance in the hydrosphere and in mineral salts such as dolomite and magnesium carbonateCommon dietary sources of magnesium include nuts cashews peanuts almonds beans bananas apples carrots broccoli and leafy greens. It has the atomic number 12 which implies that it has 12 protons and 12 electrons. Magnesium is a shiny silver or gray colored metal that is light in weight and strong.
Chemical Properties of Magnesium Magnesium is located among the alkaline earth metals on the periodic table. Magnesium is classified as an alkaline earth metal and has 2 hydration shells. Additionally no correlation between the physical state of the drug in different microspheres and their biopharmaceutical properties.
Based on the results obtained from the pure samples it appears that differences in the lubricant properties of magnesium stearate are correlated with differences in moisture content and crystalline structure. Magnesium enters in the structure of chlorophyll. Magnesium is the most chemically active element.
Magnesium is a silvery-white low density reasonably strong metal that tarnishes in air to form a thin oxide coating. Another most common method of producing MgCl 2 is by Dow process. The metal reacts with water to produce hydrogen gas.
Its compounds are widely used in construction and medicine and magnesium is one of the elements essential to all cellular life. We find Magnesium in the second position in the periodic table. The physical properties of magnesium include a melting point of 1202F 650C and a boiling point of 1994F 1091C.
Refer to the article on Magnesium Element for additional information and facts about this substance. The largest single use of magnesium is as an alloying element in aluminum alloys. What is magnesium chemical formula.
The Physical and Chemical Properties are the characteristics of a substance like Magnesium which distinguishes it from any other substance. The chemical properties of magnesium include having a tendency to react with halogens such as chlorine to form ionic salts. Articles Chemical Physical and Lubricant Properties of Magnesium Stearate KD.
What is Magnesium. Magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. Magnesium chloride in its anhydrous form is generated by heating hexamine-complex chloride salt as well.
The lubricant properties of the compound were further examined using three hydrates of laboratory-prepared pure magnesium stearate. This element belongs to the group 2 and period 3 of the periodic table. Magnesium has an atomic mass of 243 and is number 12 in the period table.
In boiling water the place of hydrogen is taken by Magnesium and a number of metals can be produced using thermal reduction of its salts and oxidized forms with magnesium. Magnesium and its alloys have very good corrosion resistance and good high temperature mechanical properties. Mg HgCl 2 MgCl 2 Hg.
Other uses include its use as a reducing agent in the production of titanium zirconium uranium beryllium and hafnium. Th density of magnesium is 1738 gmL which means the metal will sink. The most preferred method of anhydrous magnesium chloride preparation is the chemical reaction between magnesium and mercury II chloride.
Chemical Properties of Magnesium Magnesium is situated among the alkaline earth metals on the periodic table.
ads
