Today we see how much you actually know about it as we put you to the test on this quiz on Station Three. Group 1 indicates that the elements lying in that group have only 1 electron in its outermost orbit.
Difference Between Periods And Groups Difference Between
Metals on the periodic table.
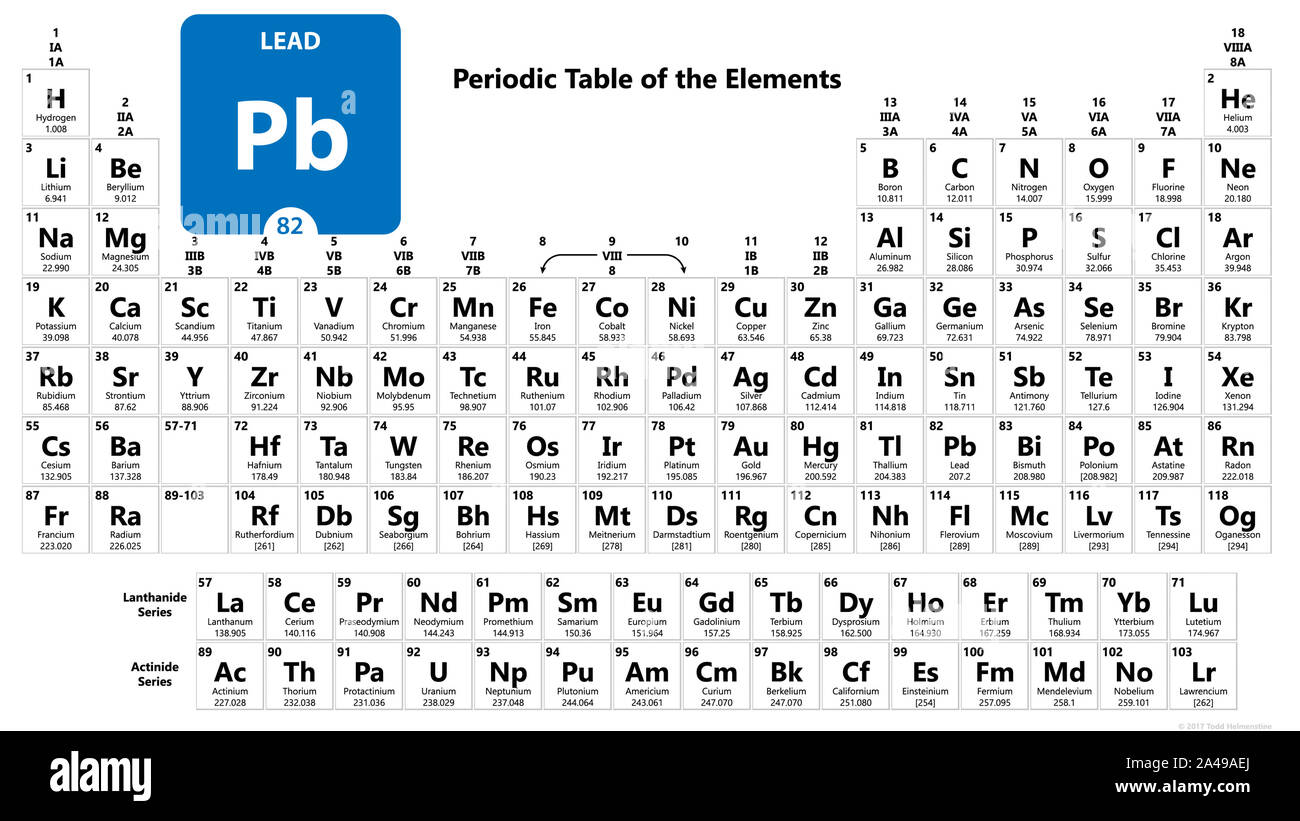
Groups and periods on the periodic table. There are seven periods in the periodic table with each one beginning at the far left. Groups and Periods on the Periodic Table. A period is a horizontal row of the periodic table.
Periodic Table Periods And Groups Practice - Displaying top 8 worksheets found for this concept. Groups and Periods are the two ways to categorize the chemically organic or inorganic elements or metals present in the modern periodic table and the key difference between group and period is its location in the periodic table. The horizontal rows are called periods.
Groups and Periods on the Periodic Table Each Period in the Periodic Table Corresponds To. For example Period 1 represents that the elements lying in that period have only 1 orbit. The periods on the Periodic table represents the number of shells or orbits of an atom.
Groups And Periods On The Periodic Table. At some point you will have heard reference to the periodic table. The vertical columns are called groups.
There are 2 elements in the first period ie hydrogen H and helium He. Groups probably come in most handy when predicting the properties of an element. The columns from the periodic table that have elements that display a.
A new period begins when a new principal energy level begins filling with electrons. Periods are horizontal rows across the periodic table while groups are vertical columns down the table. Group In chemistry a group also known as a family is a column of elements in the periodic table of the chemical elementsThere are 18 numbered groups in the periodic table but the f-block columns between groups 2 and 3 are not numbered.
Regardless of whether it was in passing or active study. The periodic table is organised in groups and periods. Here are the main features of the table.
Explain the relationship between the chemical behavior of families in the periodic table and their electron configurations. Atomic number increases as you move down a group or across a period. Members of the same group in the table have the same number of electrons in the outermost shells of their atoms and form bonds of the same type.
Group 2 represents that the elements lying in that group. Elements in the same group. These elements have only 1 electron orbit.
Groups and periods are two ways of categorizing elements in the periodic table. Just like people in a. Periods correspond to the relationship of orbitals or likely areas in which electrons will be found inside the outermost shell of the atom.
Groups are given a number to show where they are in the periodic table and also to identify the group of elements in them. The columns of the periodic table are called groups. Atomic number increases as you move down a group or across a period.
The groups are the vertical columns. To remember that groups run along the top of the table imagine a table with musical groups on top of it. Periods are horizontal rows across the periodic table while groups are vertical columns down the table.
The groups run along the top of the table while the periods run down the side. Many elements tend to be metals. The Periodic Table has 118 elements which organized on the basis of atomic number and grouped based on similarity in chemical properties.
The periodic table today is arranged with two different parts the groups and the periods. Metals on the Periodic Table. The horizontal rows are called periods.
In other words group 1 elements have 1 valence electron. The first element is Hydrogen H with atomic number 1 and the last element is Oganesson Og with atomic number 118. As you now know periods are on the horizontal line and groups are on the vertical line.
Groups and Period in the Periodic Table The periodic table of the chemical elements Columns represent Groups and Rows represent Periods. The vertical columns on the periodic table are called groups or families. Metal groups are found on the left of the table groups of non-metals are on the right.
Give the name and location of specific groups on the periodic table including alkali metals alkaline earth metals noble gases halogens and transition metals. Some of the worksheets for this concept are Anorganizedtablework due theperiodictableof Periodic table work Periodic table work 2 Unit 3 notes periodic table notes Introducing the periodic table Elements work name Periodic table review Periodic table work. Period 1 has only two elements hydrogen and helium while periods 2 and 3.
Groups and periods are two ways of categorizing elements in the periodic table. Similarly consider group 2 of the periodic table.
Both aluminium oxide Al 2 O 3 and zinc oxide ZnO are amphoteric oxides. Amphoteric oxides generally occur between the basic oxides on the left hand side of the Periodic Table and the acidic oxides on the right.
/PeriodicTableOxidation-BW-56a12da83df78cf772682bfe.png) Periodic Table Of The Elements Oxidation Numbers
Periodic Table Of The Elements Oxidation Numbers
Isotope pattern for N 2 O The chart below shows the calculated isotope pattern for the formula N2O with the most intense ion set to 100.

Oxide on the periodic table. Na 2 O and MgO. Acidic Amphoteric Basic. Can you apply this.
It is a member of the chalcogen group on the periodic table a highly reactive nonmetal and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Non-metal oxides on the right side of the periodic table produce acidic solutions eg. Isotope pattern for AgO The chart below shows the calculated isotope pattern for the formula AgO with the most intense ion set to 100.
Metal Oxides have an oxidation number of -2 and generally comprise of an oxygen anion. Isotope pattern for MgO The chart below shows the calculated isotope pattern for the formula MgO with the most intense ion set to 100. Outlines the patterns in how metals and non-metals form oxides.
Use this periodic table for calculating molar mass for any chemical formula. Covers the HSC chemistry syllabus dot point. There are three nonmetal oxides from the upper right portion of the periodic table CO NO and N2O which have such low oxidation numbers for the central atom that they give neutral aqueous solutions.
When a substance reacts chemically both as a base or acid it termed as an amphoteric solution. By mass oxygen is the third-most abundant element in the universe after hydrogen and helium. The periodic tablethe ionic compound MgO magnesium oxide.
Both sulfur and phosphorus oxides demonstrate that increasing the oxidation state increases the acidity of the oxide. Since the acidity of a cation rises rapidly with charge d-block elements which. Cl 2 O SO 2 P 4 O 10.
The table shows element percentages for N 2 O nitrous oxide. A look at the trends in oxides in the Periodic Table. Metal oxides on the left side of the periodic table produce basic solutions in water eg.
IOS app is also available. This periodic table contains the oxidation numbers of the elements. The table shows element percentages for AgO silver oxide.
Webelements periodic table iron oxide how to write the formula for iron iii sulfate you periodic table with common ionic charges what is the molar mass of iron iii oxide fe2o3 quora. The basic trend in oxide activity down the period groups of the periodic table is. Values in italics represent theoretical or unconfirmed oxidation numbers.
Oxide any of a large and important class of chemical compounds in which oxygen is combined with another element. With the exception of the lighter inert gases helium He neon Ne argon Ar and krypton Kr oxygen O forms at least one binary oxide with each of the elements. Although the compound magnesium oxide contains charged species it has no net charge because it contains equal numbers of Mg 2 and O 2 ions Likewise oxygen reacts with calcium just below magnesium in Group 2 to form CaO calcium oxide.
This table also contains the element number element symbol element name and atomic weights of each element. This is a general rule in oxides throughout the periodic table. A basic oxide is an oxide which when combined with water gives off a base.
Bold numbers represent the more common oxidation states. Whats people lookup in this blog. Options for hiding the symbol or name of the elements provide a handy learning aid for memorizing the periodic table.
Non-metals are found mostly in the top-right corner of the periodic table and thus have acidic oxides. Neutral Oxide is one which neither has an acidic characteristic or a basic one. The table shows element percentages for MgO magnesium oxide.
The Periodic Table of Oxides.
He noticed that there were groups of elements that exhibited. Comprehensive data on the chemical element Lead is provided on this page.
 Lead Definition Uses Properties Facts Britannica
Lead Definition Uses Properties Facts Britannica
Lead has the atomic number of 82.
Pb in the periodic table. 2072 1 Lead has been known since ancient times. Density at 0 Celsius. Element 82 of Periodic table is Lead with atomic number 82 atomic weight 2072.
Periodic Table of Elements Element Lead - Pb. Proud Boys PB is reveli. It has 82 protons and 82.
Density at 0 Celsius. Lead Pb a soft silvery white or grayish metal in Group 14 IVa of the periodic table. The periodic table also known as the periodic table of elements is a tabular display of the chemical elements which are arranged by atomic number electron configuration and recurring chemical properties.
The Periodic Table Periodic Law - When the elements are arranged in order of increasing atomic number there is a periodic repetition of their physical and chemical properties Modern Periodic consists of. A free detailed classroom poster of the Periodic Table. Element Lead Pb Group 14 Atomic Number 82 p-block Mass 2072.
To access this resource you need to log in or register. The structure of the table shows periodic trends. Density at 0 Celsius.
Density of the elements. Periods - Horizontal rows of the periodic table side to side Groups or families - vertical up and down column of elements in. The creator of the periodic table Dmitri Mendeleev in 1869 began collecting and sorting known properties of elements like he was playing a game while traveling by train.
Known in antiquity and believed by the alchemists to be the oldest of metals lead is highly durable and resistant to corrosion as is indicated by the continuing use of. It is a very soft highly malleable and ductile blue-white shiny metal that tarnishes in moist air but is stable in oxygen and water. Common chemical compounds are.
Why Arrange Elements in a Table. This periodic table chart lists. Pb is the chemical symbol for the element of Lead.
Tabular arrangement of the chemical elements ordered by atomic number. Density of diamond form is 3500. PB stands for Plotz and Bobkes.
Sources facts uses scarcity SRI podcasts alchemical symbols videos and images. Seeing chemical elements arranged in the modern periodic table is as familiar as seeing a map of the world but it was not always so obvious. Value given for graphite form.
84 Po Polonium 209 85 At Astatine 210 86 Rn Radon 222. Here are some words with Pb in them in case you need one to remember Pb. Including scores of properties element names in many languages most known nuclides of Lead.
You need to sign in to access free bpES resources or take advantage of. 15 rows Get the facts about element Lead Pb 82 from the periodic table. A claim echoed by the FBI and Biden but challenged by Trump.
Its a curious thing that ADL lists a Canadian comedian as head of Proud Boys while claiming ANTIFA has no leader or leadership. 82 Pb Lead 2072. The origin of the name comes from the Latin word plumbum meaning liquid silver.
Density at 0 Celsius. Lead is very malleable ductile and dense and is a poor conductor of electricity. Density at 0 Celsius.
Lead is a chemical element of the periodic table with chemical symbol Pb and has atomic number 82 with an atomic mass of 20721 u and it belongs to the element category post-transition metal. Density at 0 Celsius. 120 rows Periodic Table with Element Names and Electronegativity.
83 Bi Bismuth 20898. 7 rows Lead is a 82. Chemical element in the periodic table of elements.
Lead symbol Pb has a Face Centered Cubic structure and SlateGray color.
The groups are numbered one through 18. There are 18 Groups on the Periodic Tableand the Group number refers to the number of valence electrons.
How Many Columns Are In A Periodic Table Quora
The elements in a group share the same configuration of valence electrons which gives the elements similar chemical properties.
Columns of the periodic table. Because alkali metals __________ quickly with other elements they exist in nature only as _________. Groups 3 to 12 of the periodic table. As well as being numbered some of these groups have namesfor example alkali metals the first column of elements alkaline earth metals the second column of elements halogens the next-to-last column of elements and noble gases the last column of elements.
Groups indicate elements with similar chemical and physical properties. Also noble gas element in group 18. Group 2A of the periodic table.
Usually individual groups bear the name of the lightest metal eg Vanadium group. Elements within the same period or group have similar properties. Group 8A of the periodic table.
The groups of the periodic table are displayed as vertical columns numbered from 1 to 18. From Wikipedia the free encyclopedia In the periodic table of the elements each numbered column is a group. The Elements displayed in each Periodic Table Group are either Gas Liquid or Solid at room temperature and are classified in groups as.
Determining Chemical Properties using the Periodic Table. Group 7A of the periodic table. Elements in the same group can be expected to behave in a similar way because they have the same number of electrons in.
The older IUPAC system used Roman numerals together with letters to distinguish between the left A and right B side of the periodic table. The columns of the modern periodic table represent groups of elements and rows represent the periods. A vertical column in the periodic table.
Herein how are elements classified into groups. The periodic table is the tabular arrangement of all the chemical elements on the basis of their respective atomic numbers. Each row of the periodic table is called a period and each column of the periodic table is called a group or family.
Inner transition metal in the bottom of the bottom two rows of the periodic table. Element in group 2. The columns on the periodic table of elements are called groups.
The modern periodic table is based on the modern periodic law put forward by the English physicist Henry Moseley which states that. Some groups have specific names like the halogens or noble gases. Group 1A of the periodic table.
Element in group 17. We now know elements in the periodic table are ordered by atomic numbers. The elements in a group have very similar chemical properties which arise from the number of valence electrons presentthat is the number of electrons in the outermost shell of an atom.
The modern Periodic Table reflects electronic structure. Vertical column of the periodic table. The columns that comprise the periodic table are called groups -- 18 in total.
Here are some of the names for the various groupe columns 1st Li NaK. Element in group 1. Alkaline earth metals 3rd to 10th.
The f-block columns between groups 2 and 3 are not numbered. The number of valence electrons in a group is sometimes represented with a Roman numeral above the column. A group is a vertical column in the periodic table of the elements.
In the periodic table the vertical columns are called groups and the horizontal rows are called periods. In chemistry a group also known as a family is a column of elements in the periodic table of the chemical elements. The elements in group 1 of the periodic table are the _________ They include lithium sodium potassium rubidium cesium and francium.
Elements typically metals can lose electrons to become oxidized or typically as non-metals can gain electrons to become anions. A column of elements on the periodic table. Alkali Metals Alkaline Earth Metals Transition Metals Metalloids Other Metals Non-metals Halogens Noble Gases and Rare Earth Elements.
There are 18 numbered groups in the periodic table. Alkali metals 2nd Be Mg Ca. Element in group 16.
Columns of the periodic table typically mark groups or families. Apart from a few instances such as tellurium and iodine which have caused considerable confusion this order is the same as the order of elements relative atomic masses. Groups are considered the most important method of classifying the elements.
Three systems have been used to number families and groups. About 80 percent of the elements are metals shiny elements that conduct heat and electricity well and 15 percent of the elements are nonmetals poor conductors of heat and electricity.
The elements hydrogen and helium have a single orbital shell. Chemists use the properties of elements to sort them into groups.
 How Are Elements Organized On The Periodic Table
How Are Elements Organized On The Periodic Table
Elements are listed in numerical order by atomic number.

How are elements organized in the periodic table. The Periodic Law states that when elements are arranged in order of increasing atomic number there is a periodic repetition of their physical and chemical properties. Visualize trends 3d orbitals isotopes and mix compounds. Periodic Table of Elements.
Elements are arranged from left to right and top to bottom in order of increasing atomic number. Elements are classified as metals nonmetals and metalloids. It is also important that the elements are sorted into different categories from left to right alkali metals nonmetals metals poor metals gases and noble gases.
Study chemistrys periodic law to understand elements properties and how they relate to one. In the graphic organizer below ask a what or how question for each heading. CHEM120 OL Week 2 Lab OL Lab 3.
Each horizontal row on the periodic table is called a period. Lanthanoids and Actinoids are reactive metals. Get the table organized in time.
Representative elements include the s block and the p block of the periodic table. It also explains what information the periodic table contains. As you read write the.
What are 3 ways the periodic table is organized. Look up chemical element names symbols atomic masses and other properties visualize trends or even test your elements knowledge by playing a periodic table. The app currently retrieves information about the elements from the database and allows access to this information via a drop down menu.
Properties of elements repeat in a predictable way when atomic numbers are used to arrange elements into groups. How are all elements organized in the table. These elements are all unreactive and exist as gases.
New questions in Chemistry. Heres how it works. The American Heritage Student Science Dictionary Second Edition.
He arranged elements in the periodic table in order of increasing atomic mass. Describe the structure and organization of the periodic table Classify elements of a family based on their location in the periodic table Distinguish metals from other element classes based on typical characteristics Use the flame color test to identify metals. Its all about how many electrons an element has.
Elements and the Periodic Table. A table in which the chemical elements are arranged in order of increasing atomic number. This section explains how the elements are organized in a chart called the periodic table.
Periodic Table And Element Structure. What information does the periodic table contain. Therefore elements that have similar chemical and physical properties end up.
In terms of how reactive they are the closer it is to the bottom left corner the more reactive they are whereas the top right corner is the least reactive. They are also organized using groups similar properties. There are two rows of elements found below the main body.
Periodic table in chemistry the organized array of all the chemical elements in order of increasing atomic number. The bottom two rows of elements actually fit into the periodic table but were placed below the table to create more room. The periodic table also known as the periodic table of elements is organized so scientists can quickly discern the properties of individual elements such as their mass electron number electron configuration and their unique chemical properties.
Use Target Reading Skills Before you read preview the red headings. The table starts with the simplest atom hydrogen and then organizes the rest of the elements by atomic number which is the number of protons each contains. The horizontal across columns are called periods There are 7 periods on the periodic table.
The seven rows in the periodic table are known as the Periods. Learn about the periodic table of the elements including its history how elements are organized and how to use the table to predict properties. They are organized by the atomic number from least to greatest.
Elements are arranged in order of increasing atomic numbers. Hydrogen has 1 electron therefore it is the first in the table. The properties of elements are periodic functions of their atomic number.
In the modern periodic table elements are arranged by increasing atomic number number of protons. Each vertical column on the periodic table is called a group. The modern periodic law states.
Elements in the second row of orbitals have two orbital shells and so on. Elements with similar properties are arranged in the same column called a group and elements with the same number of electron shells are arranged in the same row called a period. Each element in a particular row has the same number of electron shells surrounding the atomic nucleus.
ads
