Metallic radii for 12-coordination are given for all metals. Copper has the fcc crystal structure.
 Materials 2021 Atomic Radius Of Copper Cu State Uses Discovery
Materials 2021 Atomic Radius Of Copper Cu State Uses Discovery
Orbital Radius pm Radius AU Periodicity link.

Atomic radius of copper. Covalent radii are in parentheses. Copper has the fcc crystal structure. The metal has a density of 896 Mgm3and a melting point of 1083 C.
Copperis a metal in group IB of the periodic table with the atomic number 29and an atomic weight of 6354. What will be the atomic radius of copper if the distance between two adjacent copper atoms in metallic copper is 256 pm. 2 1 2 3 4 a mildly basic oxide.
There are cool facts about Copper that most dont know about. General and Atomic Properties of Copper. Periodic Table Main Page.
In the case of Copper the atomic radius is 157 Å. 2551 x 10 -10 m. What is the volume of the unit cell.
All atoms ions have an ionic radius even Copper. In the case of Copper the ionic radius is 73 2 Å. Ionic radii are for six-coordination.
Atomic radius of Cerium Ce. 7 Zeilen The atomic radius of Copper atom is 132pm covalent radius. 119 Zeilen Atomic radius of Lanthanum.
128 pm Out of Sb Bi Md and Tm ____ has the smallest atomic size and ____ has the largest atomic size. You Can Visit Our Managed. Ar4s 1 3d 10.
1 Å 100pm. This Top Homework Answer is College level and belongs to the Engineering subject. Copper has FCC structure and its atomic radius is 1278.
Assuming an atomic radius of 130 pm for copper atom Cu 6354. 119 Zeilen Atomic radius. Theelectronic configuration of coper is Ar3d104s1and an atomic radius of 0128 nm.
Valence shell orbital radii for copper. All measurements given are in picometers pm. Ok so what is the ionic radius of a Copper ion.
Assuming an atomic radius of 130 pm for copper atom Cu 6354 what is the length of unit cell of. If The Atomic Radius Of Copper Is 0128 Nm Calculate The Volume Of Its Unit Cell In Cubic Meters. It must be noted atoms lack a.
140 pm Van der Waals Atomic Symbol. The average radius of copper is 135 3 pm pm its atomic radius or Bohr radius is 145 3 pm Bohr radius pm its covalent radius is 138 3 pm pm and its radius of Van der Waals is 140 3 pm pm. 1489 users searched for this homework answer last month and 68 are doing it now lets get your homework done.
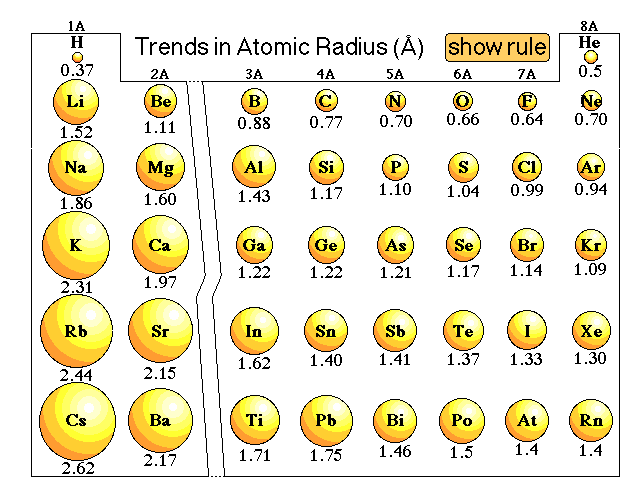
In the case of Neon the covalent radius is 071 Å. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus.
 The First Property To Explore Is Atomic Radius
The First Property To Explore Is Atomic Radius
Hence neons atomic radius must be much more than that of fluorine.

Atomic radius of neon. Although more electrons are being added to atoms they are at. Because neon and argon dont form bonds you can only measure their van der Waals radius - a case where the atom is pretty well unsquashed. Hence flourine is smaller than neon.
Neon Facts Neon Covalent Radius 071 Å Atomic Number 10 Learn more about the atomic number. All the other atoms are being measured where their atomic radius is being lessened by strong attractions. In the case of Neon the atomic radius is 051 Å.
It must be noted atoms lack a well-defined outer boundary. The reported radii of noble gas elements are van der Waals radii which are 40 more than the actual atomic radii. There are cool facts about Neon that most dont know about.
The atomic radius of Neon atom is 58pm covalent radius. Orbital Radius pm Radius AU Periodicity link. Oxygen 0 C nitrogen N D.
Because neon and argon dont form bonds you can only measure their van der Waals radius - a case where the atom is pretty well unsquashed. Atomic radii represent the sizes of isolated electrically-neutral atoms unaffected by bonding topologies. Read remaining answer here.
Molecules and atoms have a certain size or radius even Neon. You arent comparing like with like if you include the. Correct answer to the question Which of the following elements has the largest atomic radius.
Learn more about the atomic radius here. 119 Zeilen Atomic radius of Neodymium Nd 229 pm. You have to ignore the noble gas at the end of each period.
The general trend is that atomic sizes increase as one moves downwards in the Periodic Table of the Elements as electrons fill outer electron shells. All the other atoms are being measured where their atomic radius is being lessened by strong attractions. It is known that the Van der Waals radius is greater than the covalent radius.
Valence shell orbital radii for neon. 7 Zeilen The atomic radius of Neon atom is 58pm covalent radius. Trends in atomic radius across periods.
The radius of flourine is measured using covalent radius whereas that of neon is measured using Van der Waals radius. Atomic radii decrease however as one moves from left to right across the Periodic Table. State at 20 C gas.
It must be noted atoms lack a. Oxygen 0 C nitrogen N D. Ok but what is the covalent radius of an atom of Ne.
Let me show you. You see there is a lot of info about Neon beyond an atoms radius that is actually interesting. It must be noted atoms lack a well-defined outer boundary.
Why does neon not have a covalent radius. It is not possible to get covalent and metallic radii. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus.
Atomic radii of fluorine and neon in Angstrom units are respectively given by A 072 160 B 160 160 C 072 072 D None of t. The atomic radius of Neon atom is 58pm covalent radius.
ads
