Iodine is a good example for sublimation. Dry ice or solid carbon dioxide sublimes.
5 Sublimation Examples In Everyday Life
Dry ice solid CO2 provides a common example of sublimation.
What is an example of sublimation. Other examples include iodine arsenic and naphthalene moth balls. The solid carbon dioxide sublimates and becomes a gas at room temperature. As a sublimating material.
As mentioned earlier dry ice is one of the most popular examples of sublimation in real life. Examples of sublimation are channeling inappropriate urges into positive behaviors like exercise therapy or other physical activities. Sublimation ExamplesDry ice or solid carbon dioxide sublimesSnow and ice can sublime in the winter months without meltingMoth balls sublimeFrozen foods will sublime and you will find ice crystals inside of the box or bag.
Generally it is expressed as KJmol or even KJkg. Sublimation is another one of these phase transitions. An interesting one is graphite which sublimates at 3915C 4020C Langes Handbook of Chemistry 15th edition.
Through sublimation a substance changes from a solid to a gas without ever passing through a liquid phase. Iodine at a temperature of. Water can undergo sublimation under some atmospheric conditions.
Iodine is another example of a substance that undergoes sublimation. Freuds psychoanalytic theory defined sublimation as a process by which negative urges drives and behaviors are channeled into more socially acceptable behaviors. Someone with excessive sexual desires that put them in danger takes up running.
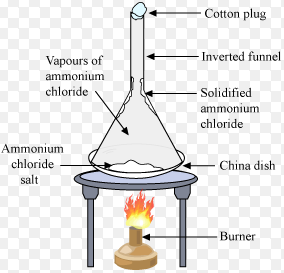
The inverse process that is the direct transition from the gaseous state to the solid-state is called inverse sublimationDry ice Solid Iodine and Ammonium Chloride are examples of Sublimation. The solid carbon dioxide sublimates and becomes a gas at room temperature. While its no surprise that ice turns to liquid upon heating frozen water can also turn to air under specific.
At room temperature and pressure it is sublimed into carbon dioxide. 20 examples of sublimation 1- Carbon dioxide. Relatively very few solids are capable of sublimation.
Sublimation is the process of the change of state from solid to gaseous state without passing through the liquid state. Sublimation Examples in Real Life Dry Ice. The best-known example of sublimation is dry ice.
Under special conditions frozen water ice can bypass the liquid phase and sublimate into the air. Dry ice is solid carbon dioxide. A person with an obsessive need for control becomes a successful administrator.
What are the defense mechanisms in psychology. Examples of sublimation in psychology A youth has anger issues so he is signed up to a local boxing club. Except in this case we have a solid turning directly into a gas.
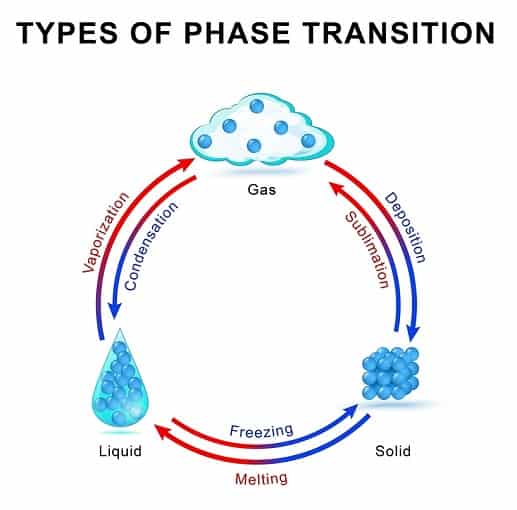
Hence this process of sublimation can be used as an excellent purification method. Sublimation is a type of phase transition or a change in a state of matter just like melting freezing and evaporation. The heat or energy required to change the state from solid to gas is called the enthalpy of sublimation and this is unique to each substance.
Its a silver-gray solid that gives off a purple gas. The best-known example of sublimation is dry ice.
Keeping this in view what are some examples of sublimation Besides dry ice. Thanks for Watching Welcome to the channel Students Study World In This Channel well teach you in Better Way.
 Sublimation Class 9 Matter In Our Surrounding
Sublimation Class 9 Matter In Our Surrounding
This endothermic phase transition occurs at temperatures and pressures below the triple point.

What is sublimation chemistry. Sublimation Definition Sublimation is the transition from the solid phase to the gas phase without passing through an intermediate liquid phase. Desublimation or deposition is the phase change from gas directly to solid with no intermediate liquid phase. Sublimation is the process of changing a solid into a gas without passing through the liquid phase.
This means that the artwork is transferred to an object in a gas state by using a high temperature heat press during the dye sublimation printing process. Most solids do not have an appreciable vapor pressure at easily accessible temperatures and for this reason the ability to sublime is uncommon. In biomedical sciences and is a science writer educator and consultant.
It differs from traditional printing methods because it bypasses the liquid step. Please Subscribe our cha. Depending on the nature of the solid sublimation can occur at atmospheric pressure or vacuum.
Sublimation is an analogous process to boiling as it occurs when a compounds vapor pressure equals its applied pressure often the atmospheric pressure. The process in which a solid transforms into a gas phasewithout first melting to form a liquid phase. Sublimation is the transition from the solid phase to the gas phase without passing through an intermediate liquid phase.
Sublimation is defined as the change or transition from the solid phase into the gas phase without entering the liquid phase. Sublimation chemistry Dark green crystals of nickelocene sublimed from the round-bottom flask and freshly deposited on a cold finger Sublimation is the transition of a substance directly from the solid to the gas phase without passing through an intermediate liquid phase. Sublimation in chemistry refers to the phase transition in which matter changes state from a solid immediately into a gas without passing through an intermediate liquid phase.
This endothermic phase transition occurs at temperatures and pressures below the triple point. Desublimation is the reverse process of sublimation. The transition of solid-phase into gaseous phase is sublimation.
When a solid material turns into a gas without going through a liquid stage. The matter does not undergo a liquid phase during this phase transition that is the solid directly turns into a gas. What is Sublimation.
Sublimation is not synonymous with evaporation. Helmenstine holds a PhD. Both sublimation and deposition phase changes are demonstrated using dry ice a copper penny and some readily available water vapor.
In the sublimation process this reaction is an endothermic reaction as the chemical bonds between molecules are broken down in order to release them into the air. Sublimation consists of the evaporation of a solid from a hot surface and subsequent condensation on another surface at a lower temperature. Sublimation occurs when atmospheric pressure is too low for a substance to exist in liquid form.
Sublimation is a type of phase transition or a change in a state of matter just like melting freezing and evaporation. The opposite process of this where the gas goes directly to the solid phase is called de-sublimation or deposition. Through sublimation a substance changes from.
Sublimation is the inverse of deposition the phase transition in which gas goes immediately to a solid. The difference is that sublimation involves a solids vapor pressure instead of a liquids. She has taught science courses at the high school college and graduate levels.
What is the definition of sublimation in chemistry. Evaporationis a liquid-to-gas phase change Frequently happens with substances. In the reverse process energy is given out.
To sublime a substance a certain energy must be transferred to the substance via heat q or work w.
ads
