This formula means that there are two hydrogen atoms and one oxygen atom in each water molecule. Hydrogen peroxide is unstable decomposing readily to oxygen and water with release of heat.
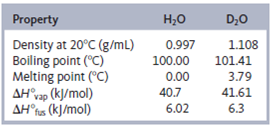 Solved Some Of The Physical Properties Of H2o And D2o Are As Foll Chegg Com
Solved Some Of The Physical Properties Of H2o And D2o Are As Foll Chegg Com
It is colorless odorless and tasteless but heavier than normal water.
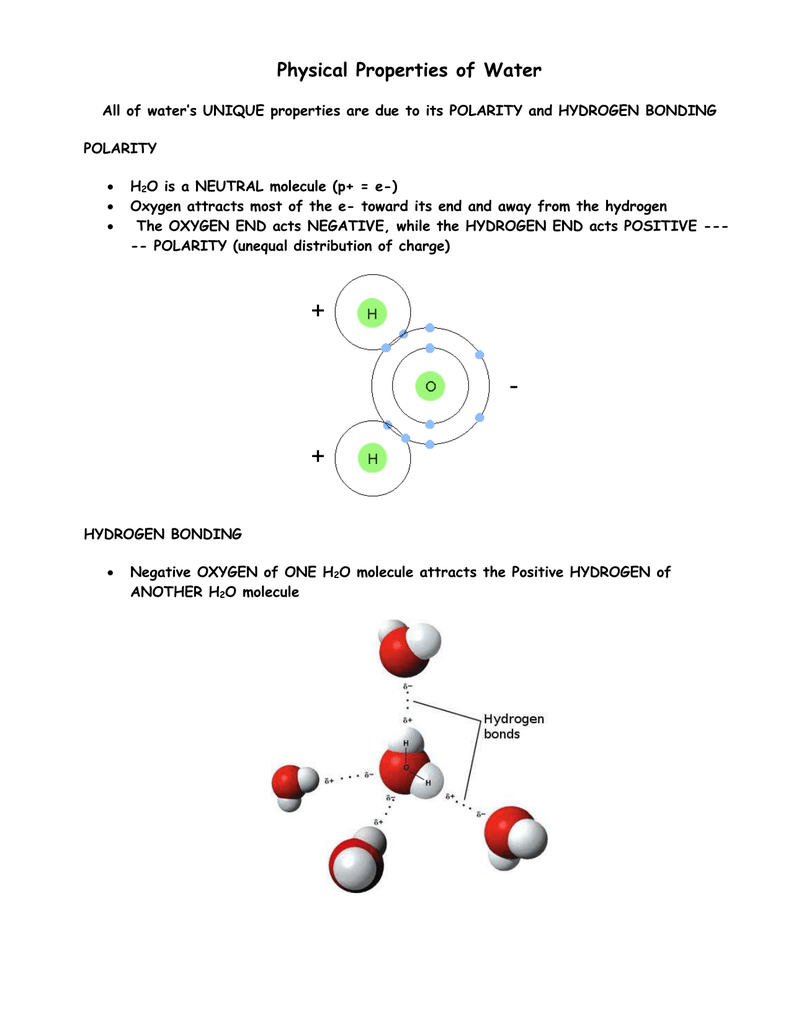
Physical properties of h2o. Physical Properties of Liquid Water Specific Heat c p BTUlbmR Thermal Conductivity k BTUhrftF Viscosity µ lbmhrft Temp F Saturated Liquid 1000 psia 2000 psia Saturated Liquid 1000 psia 2000 psia Saturated Liquid 1000 psia 2000 psia 80 09975 09943 09912 03532 03537 03570 2084 2084 2083. Cohesion is a key property of water. The slightly negative regions of one molecule are attracted to the slightly positive regions of nearby molecules forming a hydrogen bond.
In the air oxygen is simply not distinguishable since under normal conditions it is a gas without taste color and smell. There are several important properties of water that distinguish it from other molecules and make it the key compound for life. The chemical formula of water is H2O.
71 of the earths surface is made up of this liquid. It is made up of two hydrogen atoms and one oxygen atom which are held together by covalent bonds. Each water molecule can form hydrogen bonds with up to four neighbors.
But oxygen can be artificially transferred to other states of aggregation. Gas Steam or Vapor Physical States Vapor States of Water Water molecules are constantly moving Temperature increase Increase in movement Hydrological Cycle States of Water When water molecules move faster they tend to break their hydrogen bonds. However oxygen gas is colourless odourless and tasteless.
Water has a variety of unusual properties because of attractions between these polar molecules. The Physical properties of Oxygen are the characteristics that can be observed without changing the substance into another substance. Physical properties of oxygen.
Hydrogen bonds form between neighboring molecules. The molecular formula of water is H 2 O. Because of the polarity of the molecules water molecules are attracted to each other.
Properties of Saturated Water Presented at Regular Intervals of Pressure Specific volume m3kg Specific internal energy kJkg Specific enthalpy kJkg Specific entropy kJkg-K Pressure P kPa Temp. Physical properties are usually those that can be observed using our senses such as color luster freezing point boiling point melting point density hardness and odor. Ionic compounds are less soluble in heavy water because its dielectric constant is lower than that of H₂O.
The H stands for hydrogen the O stands for oxygen. The liquid and solid forms are a pale blue colour. Physical Properties The molecular mass of heavy water is higher than ordinary water which makes their properties different from each other.
Boiling Point Of Water. Although nonflammable it is a powerful oxidizing agent that can cause spontaneous combustion when it. Small amounts of gaseous hydrogen peroxide occur naturally in the air.
So at -183 o C it becomes liquid and at. T C 103 v f vg uf ug hf hg sf sg P kPa 1 69705 10001 12919 29302 23845 29303 25137 010593 89749 1. Physical Properties Oxygen exists in all three forms - liquid solid and gas.
Hydrogen peroxide is a colorless liquid at room temperature with a bitter taste.
Fluoride concentration of less than 08 10 ppm cause dental cavity tooth decay. Two atoms hydrogen H2 linked to one atom oxygen O.
 Properties Of Water Physical Chemical Properties Chemistry
Properties Of Water Physical Chemical Properties Chemistry
The properties of vinegar especially its physical qualities vary depending on the type and amount of minerals vitamins fiber and organic compound used for its production.
What is the chemical properties of water. A variety of drugs natural chemicals from foods and diseases can alter the color. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. It is one of the most plentiful and essential of compounds.
The vast majority of this water. H2O is a transparent fluid which forms the worlds streams lakes. Because of the higher electronegativity of the oxygen atom the bonds are polar covalent polar bonds.
This makes water an extremely potent solvent. Chemical properties of water. 23 реда Properties Of Water.
Blood in the urine may also turn it red. The atom electrons particles with a negative charge establish links between themselves. It undergoes several processes including alcohol fermentation and acid fermentation to assume its natural state.
Water is the chemical substance. Water molecules are polar so they form hydrogen bonds resulting in unique properties. Human urine ranges in color from nearly clear to dark amber depending largely on the amount of water that is present.
Salinity and Chemical Properties. A tasteless and odourless liquid at room temperature it has the important ability to dissolve many other substances. The chemical formula of a molecule of water is H2O.
It can form hydrogen bonds with other elements. Chemicals That Affect Urine Color. If you look at earth from space you will notice that much of the planet is covered in water.
Metal and other chemical substances in water. The molecules that attract water molecules the most are those with a full charge as an ion. Iron 03ppm excess of these cause discolouration of clothes.
The oxygen atom attracts the shared electrons of the covalent bonds to a significantly greater extent than the hydrogen atoms. Manganese 005ppm Copper 13ppm Sulphate 250 ppm Fluoride 15 ppm excess of this effects human lungs and other respiratory organs. Each molecule of water H 2 O or HOH consists of two atoms of hydrogen bonded to one atom of oxygen.
Water is a polar molecule which means it can attract other polar molecules. Physical And Chemical What is water. Vinegar is essentially a dilute aqueous solution comprised of acetic acid and water.
Oxygen is more able to keep them close to it than hydrogen. For example eating beets can turn urine red or pink harmlessly. Water a substance composed of the chemical elements hydrogen and oxygen and existing in gaseous liquid and solid states.
The level of polarity in water is extremely high uniquely so. Water is a chemical compound. 18 реда Water chemical formula.
Hence it is essential for plants. Carstensen University of Wisconsin School of.
 Chapter 3 Physical And Chemical Properties Physical And Chemical Changes Ppt Download
Chapter 3 Physical And Chemical Properties Physical And Chemical Changes Ppt Download
Physical Properties of Magnesium Edu-Resource.

Physical and chemical properties of magnesium. Introduction Physical Properties of Magnesium Oxide Chemical Properties of Magnesium Oxide Surface Structures of MgO Molecular Adsorption on MgO Bibli. This element is one of the group 2 and period 3 with the periodic table and contains the atomic number 12. Ertel x Merrell Dow Research Institute Cincinnati OH 45215 Merrell Dow Research Institute Cincinnati OH 45215 x Merrell Dow Research Institute Cincinnati OH 45215 Merrell Dow Research Institute Cincinnati OH 45215 JT.
No differences were observed in the physical state of pipemidic acid and in microsphere shape and surface between different size fractions of microspheres prepared with different amounts of magnesium stearate. Summary This chapter contains sections titled. Most common substances exist as States of Matter as solids liquids gases and plasma.
Magnesium Mg chemical element one of the alkaline-earth metals of Group 2 IIa of the periodic table and the lightest structural metal. Open image in new window. Magnesium is an important.
The element can be found in abundance in the hydrosphere and in mineral salts such as dolomite and magnesium carbonateCommon dietary sources of magnesium include nuts cashews peanuts almonds beans bananas apples carrots broccoli and leafy greens. It has the atomic number 12 which implies that it has 12 protons and 12 electrons. Magnesium is a shiny silver or gray colored metal that is light in weight and strong.
Chemical Properties of Magnesium Magnesium is located among the alkaline earth metals on the periodic table. Magnesium is classified as an alkaline earth metal and has 2 hydration shells. Additionally no correlation between the physical state of the drug in different microspheres and their biopharmaceutical properties.
Based on the results obtained from the pure samples it appears that differences in the lubricant properties of magnesium stearate are correlated with differences in moisture content and crystalline structure. Magnesium enters in the structure of chlorophyll. Magnesium is the most chemically active element.
Magnesium is a silvery-white low density reasonably strong metal that tarnishes in air to form a thin oxide coating. Another most common method of producing MgCl 2 is by Dow process. The metal reacts with water to produce hydrogen gas.
Its compounds are widely used in construction and medicine and magnesium is one of the elements essential to all cellular life. We find Magnesium in the second position in the periodic table. The physical properties of magnesium include a melting point of 1202F 650C and a boiling point of 1994F 1091C.
Refer to the article on Magnesium Element for additional information and facts about this substance. The largest single use of magnesium is as an alloying element in aluminum alloys. What is magnesium chemical formula.
The Physical and Chemical Properties are the characteristics of a substance like Magnesium which distinguishes it from any other substance. The chemical properties of magnesium include having a tendency to react with halogens such as chlorine to form ionic salts. Articles Chemical Physical and Lubricant Properties of Magnesium Stearate KD.
What is Magnesium. Magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure. Magnesium chloride in its anhydrous form is generated by heating hexamine-complex chloride salt as well.
The lubricant properties of the compound were further examined using three hydrates of laboratory-prepared pure magnesium stearate. This element belongs to the group 2 and period 3 of the periodic table. Magnesium has an atomic mass of 243 and is number 12 in the period table.
In boiling water the place of hydrogen is taken by Magnesium and a number of metals can be produced using thermal reduction of its salts and oxidized forms with magnesium. Magnesium and its alloys have very good corrosion resistance and good high temperature mechanical properties. Mg HgCl 2 MgCl 2 Hg.
Other uses include its use as a reducing agent in the production of titanium zirconium uranium beryllium and hafnium. Th density of magnesium is 1738 gmL which means the metal will sink. The most preferred method of anhydrous magnesium chloride preparation is the chemical reaction between magnesium and mercury II chloride.
Chemical Properties of Magnesium Magnesium is situated among the alkaline earth metals on the periodic table.
ads
